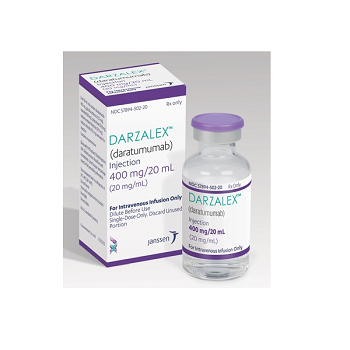
Darzalex 400mg/20ml (Daratumumab)
Courier service EMS
Other transport services
Air India Post International
Payment in the bank on the invoice
WestrUnion
MoneyGram
DARZALEX 400MG
Description
Darzalex 400mg belongs to antineoplastic drug required for multiple myeloma in patients who have getting at least 3 prior treatments.
Darzalex 400mg which is a human CD38-directed monoclonal antibody containing a proteasome inhibitor (PI) and an immunomodulatory agent or who are twice refractory to a PI and an immunomodulatory agent.
Indication
- Darzalex 400mg is indicated in combination with bortezomib and dexamethasone for the treatment of patients with multiple myeloma who have received at least 1 prior therapy
- Darzalex 400mg is indicated in combination with bortezomib, melphalan and prednisone for the treatment of patients with newly diagnosed multiple myeloma who are unsuitable for autologous stem cell transplant (ASCT)
- Darzalex 400mg is indicated in combination with lenalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at 1 prior therapy
- Darzalex 400mg is indicated as monotherapy for multiple myeloma in patients who have getting at least 3 lines of therapy, contain a proteasome inhibitor (PI) and an immunomodulatory agent (IMiD).
- Darzalex 400mg is indicated in combination with pomalidomide and dexamethasone for the treatment of patients with multiple myeloma who have received at least 2 prior therapies including lenalidomide and a proteasome inhibitor
Mechanism of action
Daratumumab belongs to a monoclonal antibody which action by producing certain cells in the immune system aggression these cancer cells. One of the antigens signify on the surface of multiple myeloma cells is called CD38. Anti-CD38 antibodies, like daratumumab, aims multiple myeloma cells by binding to the CD38 antigen and then indicating the patient’s immune system to attack the tumour.
ADME Properties
Maximum plasma concentration is 915 mcg/mL (weekly dosing)
Single treatment the volume of distribution in steady-state is 4.7 L and in combination
The half-life of Darzalex monotherapy is 18 days and combination therapy 22-23 days
Dosage and administration
Darzalex is indicated for the treatment of multiple myeloma the dosage for therapy as follows;
RECENTLY DIAGNOSED MULTIPLE MYELOMA
The Darzalex usual dose for weeks 1-6 is 16mg/kg infusion once weekly (total of 6 doses);
The Darzalex usual dose for Weeks 7-54 is 16 mg/kg IV infusion every 3 weeks (total of 16 doses)
The Darzalex usual dose for Week 55 onwards until disease progression is 16mg/kg IV infusion every 4 weeks.
RECURRENT/REFRACTORY MULTIPLE MYELOMA
Monotherapy
The Darzalex usual dose for weeks 1-8 is 16mg/kg IV infusion every 2 weeks (total of 8 doses)
The Darzalex usual dose for Weeks 9-24 is 16mg/kg IV infusion every 2 weeks (total of 8 doses)
The Darzalex usual dose for Weeks 25 onward until disease progression is 16 mg/kg IV infusion every 4 weeks.
Combination with bortezomib and dexamethasone
- The Darzalex usual dose for Weeks 1-9 is 16 mg/kg IV infusion once weekly (total of 9 doses)
- The Darzalex usual dose for Weeks 10-24 is 16 mg/kg IV infusion every 3 weeks (total of 5 doses)
- The Darzalex usual dose for Week 25 onwards until disease progression is 16 mg/kg IV infusion every 4 weeks
Combination treatment with lenalidomide and dexamethasone
- The Darzalex usual dose for Weeks 1-8 is 16 mg/kg IV infusion once weekly (total of 8 doses)
- The Darzalex usual dose for Weeks 9-24 is 16 mg/kg IV infusion every 2 weeks (total of 8 doses)
- The Darzalex usual dose for Week 25 onwards until disease progression is 16 mg/kg IV infusion every 4 weeks
Combination treatment with pomalidomide and dexamethasone
- The Darzalex usual dose for Weeks 1-8 is 16 mg/kg IV infusion once weekly
- The Darzalex usual dose for Weeks 9-24 is 16 mg/kg IV infusion every 2 weeks
- The Darzalex usual dose for Week 25 onwards until disease progression is 16 mg/kg IV infusion every 4 weeks
Side effects
Darzalex 400mg may causes some common and serious side effects as follows
Diarrhae
Throat irritation
Cough
Shortness of breath
Chills vomiting
Back pain
Joint pain
Loss of appetite
Low blood counts
Fatigue
Nasal congestion
High blood pressure
Muscular chest pain
Drug interaction
The drug Darzalex 400mg has no drug interaction studies have been performed
Contraindication
The drug Darzalex 400mg is contraindicated patients with hypersensitivity to the active substance or to any of the excipients
Precautions
Darzalex 400mg may have increased neutropenia and/or thrombocytopenia induced by background therapy; monitor CBC counts periodically during treatment; regulate patients with neutropenia for signs of infection; dose delay may be necessary to allow recovery of neutrophils; no dose reduction is recommended, consider supportive care with growth factors and/or transfusions
Darzalex 400mg will connect to CD38 on RBCs and may results in a positive indirect antiglobulin test (Coombs test)
Darzalex 400mg will cause false-positive results with serum protein electrophoresis (SPE) and immunofixation (IFE) assays
Pregnancy
During pregnancy using of drug Darzalex 400mg is not advisable due to drug mechanism of action, may cause fetal myeloid or lymphoid-cell depletion and reduced bone density.
Lactation
Excretion into human milk is unknown. May be possible adverse reaction on the breast-fed child from Darzalex 400mg
Storage
Store the drug at 20C – 80C
Avoid freezing or shake
Protect from light
Missed dose
If dose is missed then take it immediately soon, if time reach for next dose, then skip missed dose and continue regular schedule. Avoid taking two doses at a time.
- Trade name Darzalex 400mg/20ml
- Substance Daratumumab
- Manufacturer Janssen Biotech
- Packaging 20ml injection in a vial
- Country of origin India
-
Convenient deliveryWe deliver to all countries of the World *. We cooperate only with trusted cargo carriers.
-
CONTROL AND INSPECTIONItem is checked before shipping! All the medicines are delivered directly from the manufacturer and sent from India to the client's address.
-
CHOOSE USWe provide the best price, including insurance against loss through the fault of the carrier. Our company cooperates directly with the largest manufacturers of medicines in India.
-
Promotions and DiscountsWe offer a flexible system of discounts for our customers and fixed discounts for current offers. Regular customers receive a discount of up to 10% of the amount of orders, as well as a remuneration system for referring customers via a referral link.
Запазете любимите си елементи към отметки
Това няма да загуби желаната опция!