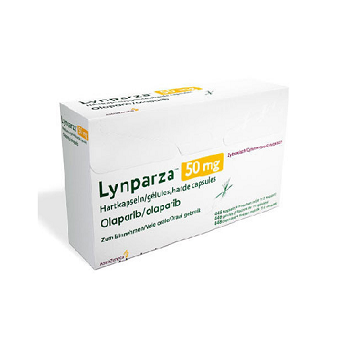
Lynparza 50mg (Olaparib)
Courier service EMS
Other transport services
Air India Post International
Payment in the bank on the invoice
WestrUnion
MoneyGram
LYNPARZA 50MG
Description
Lynparza is feasible as oral tablets marketed and was started indicated as a maintenance treatment or monotherapy for the therapy of adult patients with relapsed epithelial ovarian, fallopian tube or primary peritoneal cancer
Lynparza is a prescription drug which used under the supervision of medical practioners
Indication
Lynparza is used for the treatment in patients with advanced ovarian cancer
Lynparza is used for the treatment in patients with HER2 – negative metastatic breast cancer.
Mechanism of action
PARP is small for Poly (ADP-ribose) polymerase. It is a protein whichproduced damaged cells to repair themselves. Olaparib blocks PARP working.
Some cancer cells commit on PARP to keep their DNA healthy. This contains cancer cells with a convert in the BRCA gene. So, when Olaparib blocks PARP from repairing DNA injury, the cancer cells die.
This kind of drug is called a cancer growth blocker.
ADME Properties
Absorption: Maximum plasma concentration 1 to 3 hours
Distribution: volume of distribution 167 +/-196 L, plasma protein binding is 82%
Metabolism: Primarily metabolised by CYP3A4
Elimination: Excreted through the urine 44% and 42% through the feces.
Half – life of Lynparza 11.9 hours
Dosage and administration
Ovarian cancer
Maintenance treatment of recurrent ovarian cancer
The tablet recommended dose is 300mg (two 150mg – mg tablets) PO BID
Continue treatment until disease progression or unacceptable toxicity
Monotherapy for advanced BRCA – mutated ovarian cancer
The tablet recommended dose is 300mg (two 150mg – mg tablets) PO BID
The capsules recommended dose is 400mg (eight 50mg- mg capsules) PO BID
Continue treatment until disease progression or unacceptable toxicity.
Maintenance treatment for advanced BRCA-mutated ovarian cancer
The tablet recommended dose is 300mg (two 150mg – mg tablets) PO BID
Follow the treatment until disease progression, undesirable toxicity or completion of 2 years of treatment
Finishing of 2 years of treatment
- Patients with full response (no radiologic evidence): discontinue treatment
- Patients with indication of disease and may asset from continuous treatment: cure beyond 2 years.
Side effects
Common side effects for patients taking Olaparib
- Musculoskeletal pain
- Diarrhea
- Decreased platelet count
- Decreased Haemoglobin
- Nausea
- Decreased white blood cell count
- Abdominal pain
- Vomiting
- Upper respiratory tract infection
- Anemia
- Decreased neutrophils
- Fatigue (including weakness)
- Increased serum creatinine
Less common side effects of patients administrating Olaparib
- Vertigo
- Constipation
- Urinary tract infection
- Heartburn
- Decreased appetite
- Shortness of breath
- Myalgia
- Headache
- Skin rash
- Back pain
- Taste changes
- Cough
- Swelling
Drug interaction
When Lynparza co administrationwith myelosuppressive anticancer agents contains DNA damaging agents, indicate a potentiation and prolongation of myelosuppressive toxicity.
Interaction of strong CYP3A inhibitors (itraconazole, telithromycin, clarithromycin,) will increase Olaparib plasma concentration.
Co administration of strong CYP3A inducers (itraconazole, telithromycin, clarithromycin,) will decrease Olaparib plasma concentration.
Contraindication
None
Precautions
Haematological toxicity will be resulted in patientsincluding diagnoses or findings of commonly mild or moderate anaemia, neutropenia, thrombocytopenia and lymphopenia.
When Lynparza monotherapy administrated to patient the incidence of myelodysplastic syndrome will occur.
On basis of mechanism of action (PARP inhibition), Lynparza could cause risk to fetal when administered to a pregnant woman.
Lynparza combination with strong or moderate CYP3A inhibitors is not recommended.
Pregnancy
Based on Lynparza mechanism of action, the drug causes risk to the fetal may occur when administrated to a pregnant woman.
Lactation
No data available on the presence of Lynparza in human milk, hence avoid breast feeding during the treatment.
Storage
Store at 200C to 250C
Missed dose
If dose is failed, then have the dose as soon as possible before next dose timing reaches or skip the missed dose and continue the regular schedule.
Must Consult doctors regarding missed dose.
- Trade name Lynparza 50mg
- Substance Olaparib
- Manufacturer Myriad genetic Laboratories
- Packaging 112 capsules
- Country of origin India
-
Convenient deliveryWe deliver to all countries of the World *. We cooperate only with trusted cargo carriers.
-
CONTROL AND INSPECTIONItem is checked before shipping! All the medicines are delivered directly from the manufacturer and sent from India to the client's address.
-
CHOOSE USWe provide the best price, including insurance against loss through the fault of the carrier. Our company cooperates directly with the largest manufacturers of medicines in India.
-
Promotions and DiscountsWe offer a flexible system of discounts for our customers and fixed discounts for current offers. Regular customers receive a discount of up to 10% of the amount of orders, as well as a remuneration system for referring customers via a referral link.
Запазете любимите си елементи към отметки
Това няма да загуби желаната опция!