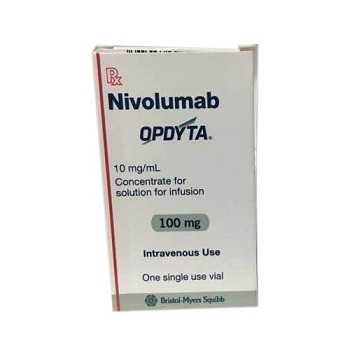
Opdyta 100mg (Nivolumab)
Courier service EMS
Other transport services
Air India Post International
Payment in the bank on the invoice
WestrUnion
MoneyGram
OPDYTA 100MG
Description
Opdyta 100mg is a checkpoint inhibitor and a type of immunotherapy which helps make cancer cells more vulnerable to attack by your body’s own immune system
An antibody which encourages the tumor-killing effects of T cells (white blood cells that help your body fight disease)
Opdyta 100mg is prescription drug which is used under supervision of doctor
Indication
Opdyta 100mg is used for the treatment of patients having Melanoma
Opdyta 100mg is used for the treatment of patients having Non-Small Cell Lung Cancer
Opdyta 100mg is used for the treatment of patients having Kidney (Renal Cell) Cancer
Mechanism of action
Nivolumab stops the activity of a molecule knowns as PD-1, a protein which prohibits T cells from identify and attacking inflamed tissues and cancer cells. PD-1 can deceit your immune system into overlooking melanoma cells as normal cells.
Nivolumab prompt your immune system’s response to melanoma by inhibiting the PD-1 protein on T cells.
Nivolumab stimulate T cells so that they can attack melanoma cells anywhere in your body.
ADME Properties
The time to peak plasma concentration is between 1-4 hours
Volume of distribution is reported to be 8L
There is no information regarding the plasma protein bounding
Half-life elimination of Opdyta is 26.7 days.
Dosage and administration
Opdyta recommended dosage for unresectable or metastatic melanoma
As single agent is either 240mg every 2 weeks or 480mg every 4 weeks given as IV infusion over 30 minutes until disease progression or unacceptable toxicity
Concomitant use with ipilimumab. The prescribed dose of Opdyta is 1mg/kg administrated as an intravenous infusion over 30 minutes, followed by ipilimumab 3mg/kg given as IV over 90 minutes on the same day. Duration 3 weeks of max 4 doses, after completing combination dose continue the single agent treatment
For adjuvant treatment of melanoma: As single agent is either 240mg every 2 weeks or 480mg every 4 weeks given as IV infusion over 30 minutes until disease progression or unacceptable toxicity for up to 1 year.
For Non-small cell lung cancer: As single agent is either 240mg every 2 weeks or 480mg every 4 weeks given as IV infusion over 30 minutes until disease progression or unacceptable toxicity.
For small cell lung cancer; As single agent is either 240mg every 2 weeks given as IV infusion over 30 minutes until disease progression or unacceptable toxicity for up to 1 year.
For renal cell carcinoma: As single agent is either 240mg every 2 weeks or 480mg every 4 weeks given as IV infusion over 30 minutes until disease progression or unacceptable toxicity.
With ipilimumab
Opdyta of 3mg/kg administrated as IV infusion over 30 minutes and followed by 1mg/kg administrated as IV infusion over 30minutes on the same day. Duration 3 weeks of max 4 doses, after completing combination dose continue the single agent treatment.
Side effects
Common side effects of Opdyta 100mg
Lymphocytopenia (Low White Blood Cells)
Less common side effects Opdyta 100mg
Colitis
Increased serum alkaline phosphatase
Drug interaction
No formal pharmacokinetic drug interaction studies
Precautions
While using Opdyta 100mg, Immune-mediated hepatitis observed in clinical trials; check for difference in liver function; withhold for moderate and permanently stop for severe or life-threatening transaminase or total bilirubin elevation
While using Opdyta 100mg Immune-mediated pneumonitis may appear to the patients; withhold for moderate and permanently discontinue for serious or life-threatening pneumonitis
While using Opdyta 100mg Other clinically significant and possible fatal immune-mediated side effects (eg, myocarditis, rhabdomyolysis, myositis, uveitis, iritis, pancreatitis) can appear after therapy discontinuation
While using Opdyta 100mg Serious infusion reactions resulted (rare, <1%); stop if severe or life-threatening; break or slow rate of infusion in patients with mild or moderate infusion reactions
While using Opdyta 100mg Immune-mediated colitis resulted; withhold for moderate or serious and permanently stop for life-threatening colitis
While using Opdyta 100mg Immune-mediated hepatitis seen in clinical trials; check for liver function changes; withhold for limited and permanently stop for serious or life-threatening transaminase or total bilirubin elevation.
While using Opdyta 100mg It may cause fetal harm; advise of possible risk to a foetus and use of effective contraception
Pregnancy
The drug-associated risk has no data available. Advise pregnant women of the potential risk to a foetus. Since the effects of Opdyta 100mg are expected to be greater during the second and third trimesters of pregnancy.
Lactation
Opdyta 100mg Excretion in human breast milk is not known; advise women to discontinue breastfeeding during treatment
Storage
Store at under refrigeration at 20C to 80C
Missed dose
If you failed a dose take it as soon as possible, if time reach for next dose, then skip missed dose and continue regular schedule. Avoid taking two doses at a time. Avoid taking missed dose within 12hrs of the next dose.
- Trade name Opdyta 100mg
- Substance Nivolumab
- Manufacturer BMS India Pvt Ltd
- Packaging 1 injection in 1 vial
- Country of origin India
-
Convenient deliveryWe deliver to all countries of the World *. We cooperate only with trusted cargo carriers.
-
CONTROL AND INSPECTIONItem is checked before shipping! All the medicines are delivered directly from the manufacturer and sent from India to the client's address.
-
CHOOSE USWe provide the best price, including insurance against loss through the fault of the carrier. Our company cooperates directly with the largest manufacturers of medicines in India.
-
Promotions and DiscountsWe offer a flexible system of discounts for our customers and fixed discounts for current offers. Regular customers receive a discount of up to 10% of the amount of orders, as well as a remuneration system for referring customers via a referral link.