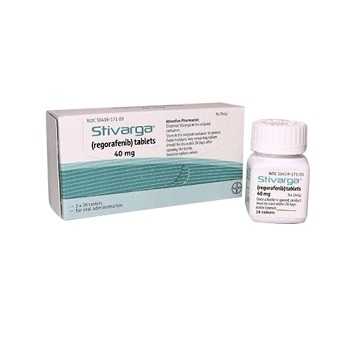
Stivarga 40mg (Regorafenib )
Courier service EMS
Other transport services
Air India Post International
Payment in the bank on the invoice
WestrUnion
MoneyGram
STIVARGA 40 MG
Description
Stivarga 40mg is a cancer medicine whichinhibits the growth and spread of cancer cells in the body.
Stivarga 40mg is usually administer after other cancer medications have been tried without success.
Stivarga 40mg is a prescription drugs which is used under the supervision of medical practioners.
Indication
Stivarga 40mg is indicated for the treatment following conditions
Hepatocellular carcinoma
Colorectal cancer
Gastrointestinal stromal tumor
Dosage and administration
Hepatocellular carcinoma
The prescribed dose is 160mg (four 40mg tablets) Po q Day for the first 21 days of each 28-day cycle.
Follow the treatment of this conditions until disease progression or undesirable toxicity
Colorectal cancer
The usual dose is 160mg (four 40mg tablets) Po q Day for the first 21 days of each 28-day cycle.
Gastrointestinal stromal tumor
The usual dose is 160mg (four 40mg tablets) Po q Day for the first 21 days of each 28-day cycle.
Mechanism of action
Regorafenib belongs to targeted cancer drug. It works by preventing proteins on cancer cells which encourages the cancer to develop. These proteins are called protein kinases. Therefore, regorafenib is called a protein kinase inhibitor (TKI) or cancer growth blocker.
It also inhibits the cancer cells from growing blood vessels which they required. Preventing blood vessel growth is called anti-angiogenesis treatment.
Regorafenib may decrease the cancer or stops it growing for a time.
ADME Properties
Time to peak plasma is 4 hours and concentration is 2.5mcg/mL for single dose; 3.9mcg/mL for steady state and bioavailability is 69-83%
Regorafenib has protein bounding about 99.5% and M-2 active metabolite is 99.8%; M-5 active metabolite is 99.95%
Regorafenib is metabolized by CYP3A4 and UGT1A9
Excreted via feces 71%; 19% urine (within 12 days of single dose)
Half-life of
Regorafenib is 28 hr
M-2 active metabolite is 25 hr
M-5 active metabolite is 51hr
Side effects
Common side effects occurring in greater than 30%
- High bilirubin in the blood
- Hand-foot syndrome
- Diarrhea
- Low platelets
- Mouth sores/inflammation
- Weight loss
- Infection
- Anemia
- Increased liver enzymes (AST, ALT)
- Fatigue
- High blood pressure
- Protein in the urine
- Low calcium
- Low phosphorous
- Low white blood cells
- Decreased appetite
- Increased lipase& amylase
- Voice disorder (Dysphonia)
- Low sodium
- Nausea
Less common side effects occurring in about 10-29% of patients receiving Stivarga 40mg
Drug interaction
Interaction of Stivarga 40mg with strong CYP3A4 inducers will decrease Regorafenib plasma concentrations and increased plasma concentration of active metabolite M-2; M-5.
Interaction of Stivarga 40mg with strong CYP3A4 inhibitor will increase Regorafenib plasma concentration and decreased plasma concentration of active metabolite M-2; M-5.
Interaction of Stivarga 40mg with BCRP substrate will increased the plasma concentration of the BCRP substrate.
Precautions
- While therapy with Stivarga will have high risk for HFSR/PPES and rash; a more incidence of HFSR reported in Asian patients; stop and then reduce or stop regorafenib depending on severity and persistence of dermatologic toxicity
- When administrating Stivarga 40mg will occur Myocardial ischemia and infarction seen in clinical trials; withhold Stivarga for new or acute cardiac ischemia/infarction and restart only after resolution of acute ischemic events
- While therapy with Stivarga, one case report of reversible posterior leukoencephalopathy syndrome (RPLS) occurred (1 of 1100 treated patients); discontinue therapy if RPLS occurs
- Severe drug-induced liver injury with fatal outcome appeared in Stivarga-treated patients in clinical trials. Some of the cases, liver dysfunction occurred within the first 2 months of treatment and was characterized by a hepatocellular pattern of injury.
- While treatment with Stivarga will have heavy risk for haemorrhage; stop therapy for severe or life-threatening haemorrhage
- While therapy with Stivarga will Increased risk of infections reported; most common infections includes urinary tract infections, nasopharyngitis, mucocutaneous and systemic fungal infections and pneumonia
Pregnancy
There are no data available on use in pregnant women. Advise female about the possible hazard to a foetus.
Lactation
Excretion into human milk is unknown
Avoid breast feeding during the treatment with Stivarga 40mg
Storage
Store the Stivarga 40mg at 250C (770C)
Keep the drug in its original container bottle
Keep the bottle tightly closed after one time used
Missed dose
If dose is missed, patients must consult with medical practitioner and follow the instructions given by them.
thereby missed dose should be avoid and follow the regular dosing schedule
- Trade name Stivarga 40mg
- Substance Regorafenib
- Manufacturer Bayer Pharmaceuticals Pvt
- Packaging 84 tablets
- Country of origin India
-
Convenient deliveryWe deliver to all countries of the World *. We cooperate only with trusted cargo carriers.
-
CONTROL AND INSPECTIONItem is checked before shipping! All the medicines are delivered directly from the manufacturer and sent from India to the client's address.
-
CHOOSE USWe provide the best price, including insurance against loss through the fault of the carrier. Our company cooperates directly with the largest manufacturers of medicines in India.
-
Promotions and DiscountsWe offer a flexible system of discounts for our customers and fixed discounts for current offers. Regular customers receive a discount of up to 10% of the amount of orders, as well as a remuneration system for referring customers via a referral link.
Запазете любимите си елементи към отметки
Това няма да загуби желаната опция!