Tagrisso 40mg (Osimeritinib)
Courier service EMS
Other transport services
Air India Post International
Payment in the bank on the invoice
WestrUnion
MoneyGram
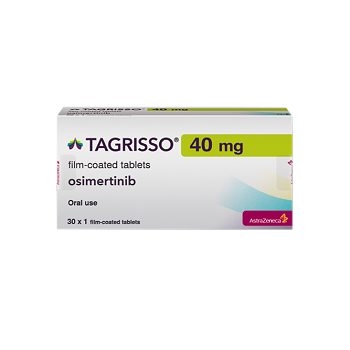
TAGRISSO 40MG
Tagrisso 40mg defines
A Tagrisso 40mg tablet is enclosing a functioning substance known as Osimeritinib, which is in the past known as Mereletinib.
Osimeritinib is an inescapable, specifically veered off epidermal development factor receptor prohibitor, containing hostile to malignancy movement.
The pharmacological class of Tagrisso 40mg is tyrosine kinase prohibitor. Osimeritinib is expressed as third generation medicine, which is accessible in tablet shape.
Tagrisso 40mg indication
A Tagrisso 40mg tablet is widely used as first line treatment for advanced non-small cell lung cancer with EGFR mutation positive patients.
Tagrisso 40mg is also indicated for the treatment of patients affected with advanced EGFR T792M mutation positive NSCLC, disease has advanced on or after the tyrosine kinase inhibitor treatment
Tagrisso 40mgPharmacological action
Osimeritinib is comparative in real life when contrasted with other tyrosine kinase inhibitor drugs.
Osimeritinib is named tyrosine kinase inhibitor of epidermal improvement factor receptor which is accessible on the surface of tumor cells
Osimeritinib is non-reversibly merge with mutant kind of EGFR at 9 folds than wild sort
Precludes EGFR honing changes exon 19 Del and L858R
Prompts denies changed EGFR with T790M restriction change
Finally, cut down activity against wild kind EGFR
Tagrisso 40mg undergoes ADME
After oral administration of Tagrisso 40mg tablets, the maximum plasma concentration time reaches within 6 hours.
The volume of distribution of Osimeritinib 918L
Osimeritinib is highly bound to human plasma protein at 95%.
Metabolism of Osimeritinib occurs via oxidation, there are two major metabolites of Osimeritinib are AZ7550 & AZ5104.
The terminal half-life period of Osimeritinib 48 hours
The clearance value of Osimeritinib is 14.3L/hr.
Nearly 68% of metabolites are eliminated through feces; 14% via urine.
Tagrisso 40mg dosage management
The prescribed dose of Tagrisso tablet is, 80mg should be administered orally as once daily.
Avoid concomitant with strong CYP3A4 inducers
In case of co administration, increase the dose of Tagrisso as 160mg. it should be taken as a single dose
Then decreased the dose of Tagrisso to 80mg 3 weeks after discontinuation of CYP3A4 inducers
Tagrisso tablet should be taken with or without food
Patient are not able to take orally, tablets must be dissolved in water and drink it immediately.
Do not break, chew or crush the Tagrisso tablets.
Stop the therapy during the conditions like
QT prolongation
Symptomatic congestive heart failure
Interstitial lung disease
In pain management
Category III or severe: Restrained the Tagrisso 40mg tablet for 3 weeks
Category 0 to II: follow at 80mg or 40mg as a single dose
If no development in 3 weeks: Break off the therapy.
Concurrent use of Tagrisso with CYP3A4 inducers, the dosage of Tagrisso should be elevates to 160mg as a single dose and ensuing by 80mg for 3 weeks after discontinuation of strong CYP3A4 inducers.
Tagrisso 40mg side effects
The most common adverse effects occurred during the therapy
Cardiomyopathy
QT prolongation
Interstitial lung disease
Keratitis
Other common side effects
Diarrhea or constipation
Hyperglycemia
Hypomagnesemia
Hyponatremia
Elevation of AST & ALT
Stomatitis
Nausea
Vomiting
Rash
Nail toxicity
Pruritus
Dry skin
Headache
Cough
Dyspnea
Fatigue
Pyrexia
Loss of appetite
Respiratory tract infection
Lymphopenia
Thrombocytopenia
Anemia
Neutropenia
Tagrisso 40mg drugs Interaction
Tagrisso 40mg tablets concomitant with strong CYP3A4 inducers, leads to reducing the exposure of Osimeritinib, this may causes reducing the effect of Tagrisso 40mg.
Avoid the concurrent use of strong CYP3A4 inducers with Tagrisso 40mg tablets.
Tagrisso 40mg tablet concomitant with BCRP substrates may causes elevating the exposure of BCRP substrates. Thus, results as raising the adverse effects related to BCRP substrates
Avoid concurrent use of Tagrisso 40mg tablets with drug which activate the QT prolongation. In this case patients should be experienced with periodic ECG monitoring.
Tagrisso 40mg usage in
Pregnancy
Pregnancy category of Osimeritinib is not designate
While using Tagrisso 40mg tablet causes embryo fetal harm
Lactation
Milk feeding should not be recommended during lactation period.
Pediatric
The efficacy of Osimeritinib in pediatric patients has not been assessed.
Tagrisso 40mg warning
Some of the adverse effects occur during the therapy
Interstitial lung disease: To avoid this problem, withheld or discontinue the Tagrisso 40mg tablets.
Keratitis: Monitor the manifestation of keratitis frequently and provide supportive measures
Embryo fetal toxicity:Tagrisso 40mg tablet used in pregnancy period causes fetal damage.
QTc extension: Avoid concurrent use of Tagrisso 40mg with drug prolong the QTc
Cardiomyopathy: Periodic cardiac monitoring is assessed; in this condition discontinue the therapy.
Tagrisso 40mg missed dose
In case of missed dose, do not take the missed dose and follow the regular dosing schedule.
Tagrisso 40mg contraindication
Contraindication is not occurred during the treatment.
In some patients, anaphylactic reactions may occur due to patients may contraindicated to the component of Tagrisso 40mg tablet.
Tagrisso 40mg storage
The storage condition of Tagrisso 40mg tablet is, stored at 25oC.
Protect the tablet carton from moisture, heat and light.
- Trade name Tagrisso 40mg
- Substance Osimeritinib
- Manufacturer AstraZeneca
- Packaging 30 tablets
- Country of origin India
-
Convenient deliveryWe deliver to all countries of the World *. We cooperate only with trusted cargo carriers.
-
CONTROL AND INSPECTIONItem is checked before shipping! All the medicines are delivered directly from the manufacturer and sent from India to the client's address.
-
CHOOSE USWe provide the best price, including insurance against loss through the fault of the carrier. Our company cooperates directly with the largest manufacturers of medicines in India.
-
Promotions and DiscountsWe offer a flexible system of discounts for our customers and fixed discounts for current offers. Regular customers receive a discount of up to 10% of the amount of orders, as well as a remuneration system for referring customers via a referral link.
Запазете любимите си елементи към отметки
Това няма да загуби желаната опция!