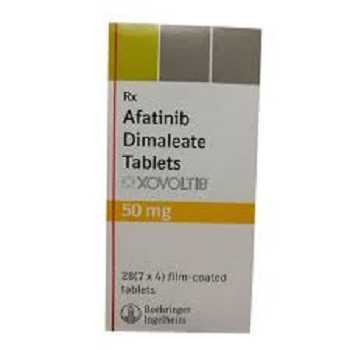
Xovoltib 50mg (Afatinib)
Courier service EMS
Other transport services
Air India Post International
Payment in the bank on the invoice
WestrUnion
MoneyGram
XOVOLTIB 50MG
Description
Afatinib is a generic form of brand name Xovoltib 50mg, an orally bioavailable anilino-quinazoline derivative and inhibitor of the receptor tyrosine kinase (RTK) epidermal growth factor receptor (ErbB; EGFR) family, with antineoplastic activity.
Xovoltib 50mg is a targeted therapy drug. Xovoltib 50mg is a prescription drugs which is used under the supervision of medical practioners.
Indication
Xovoltib 50mg is indicated for the first-line treatment for patients with metastatic non-small cell lung cancer (NSCLC)
Dosage and administration
For adult
The recommended dose for adult patients with metastatic non-small cell lung cancer is 40mg taken once per day.
For child
Dosage ages of 0-17 years is not being studied in children.
The drug should not be used age under 18 years of age
For renal impairment: prescribed dose is 30mg taken once per day
Administer the drug Xovoltib on an empty stomach. Consume the drug 1 hour before or 2 hours after a meal
Avoid cutting or crushing the tablet
Overdosage
overdose is resulted in patients whom ingested 360mg of Xovoltib which resulted in nausea, vomiting, asthenia, abdominal pain and hence provided supportive measurement for recovery.
Mechanism of action
Afatinib is a kind of cancer growth blocker called a protein tyrosine kinase inhibitor (TKI). Tyrosine kinases are proteins which trigger cells to grow. Afatinib inhibits tyrosine kinases and blocks epidermal growth factor receptor proteins in cancer cells. So Afatinib is also called an EGFR-TK inhibitor.
ADME Properties
Absorption: High plasma time is 2-5 hr and bioavailability are 92 %
Distribution: Human plasma protein bound is 95%
Metabolism: The major circulating metabolites are covalent adducts to proteins.
Elimination: The drug excretion through feces is 85% and urine 4%
Half-life is 37 hr.
Side effects
Common side effects caused
- Dry skin
- reduced appetite
- Nausea
- Vomiting
- Nail infection
- Diarrhoea
- Mouth sores
- Acne
- Itchiness
- Rash
Serious side effects
- Urine colour changes to dark
- Bleeding or bruising
- Weakness
- Blurred vision
- Rapid heartbeat
- Breathing trouble
- Cough and fever
- Yellowing the skins
Drug interaction
Xovoltib 50mg concomitant use with P-gp inhibitors (cyclosporine A, ketoconazole verapamil) with Xovoltib 50mg will have high exposure to Afatinib
When combination of Xovoltib 50mg with P-gp inducers (carbamazepine, phenytoin, phenobarbital) with Xovoltib 50mg will reduces the exposure to Afatinib
Precautions
The drug may cause diarrhea which leads in dehydration with or without renal impairment; some stated cases were fatal; withhold treatment for serious and extended diarrhea not responsive to antidiarrheal agents
Hepatotoxicity resulted; monitor with periodic liver testing; withhold or stop treatment for serious or worsening liver tests
The drug may result in Interstitial lung disease (ILD)
Embryo-fetal toxicity: Xovoltib 50mg will have risk to the foetus when administrated to pregnant women
Keratitis: if patients confirm with suspected keratitis or ulcerative keratitis then stop or discontinue the drug
May have high risk for sunburn/phototoxicity; may aggravate rash or acne; patients use with caution to limit sun exposure and where sunscreen and protective clothing
Pregnancy
While on treatment with Xovoltib 50mg Pregnancy should avoid because the drug Xovoltib 50mg is administrated to a pregnant woman will harm the foetus.
Lactation
Xovoltib 50mg Excretion into human milk is unknown or will have adverse reaction in a child who is breastfed. Therefore, decision should take whether to stop breastfeeding or discontinuation of medicine.
Storage
Xovoltib 50mg Store at 250C
Keep Xovoltib 50mg away from children
Protect away free from moisture and light
Missed Dose
If patient forget to take the dose of Xovoltib 50mg, then the missed dose must be skipped and follow the regular dosing schedule. Do not have double the dose
Must consult with medical oncologist and follow the suggestions
- Trade name Xovoltib 50mg
- Substance Afatinib
- Manufacturer Glaxo smithkline
- Packaging 7 tablets
- Country of origin India
-
Convenient deliveryWe deliver to all countries of the World *. We cooperate only with trusted cargo carriers.
-
CONTROL AND INSPECTIONItem is checked before shipping! All the medicines are delivered directly from the manufacturer and sent from India to the client's address.
-
CHOOSE USWe provide the best price, including insurance against loss through the fault of the carrier. Our company cooperates directly with the largest manufacturers of medicines in India.
-
Promotions and DiscountsWe offer a flexible system of discounts for our customers and fixed discounts for current offers. Regular customers receive a discount of up to 10% of the amount of orders, as well as a remuneration system for referring customers via a referral link.
Запазете любимите си елементи към отметки
Това няма да загуби желаната опция!